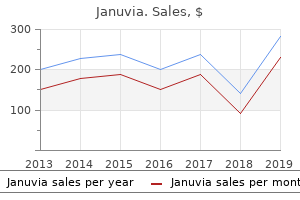
Januvia
"100 mg januvia with amex, blood glucose non fasting".
By: K. Jens, M.B. B.CH. B.A.O., M.B.B.Ch., Ph.D.
Co-Director, California University of Science and Medicine
Such signal transduction is an additional important function of adhesive junctions (12) managing of diabetes discount januvia. The principal function of the tight junctions is to occlude the paracellular channels of epithelia diabetes prevention workshop cheap januvia 100 mg online, thereby restricting intercellular leakage of molecules between the distinct biological compartments separated by the epithelium (13). Tight junctions are also important in forming tight seals between some endothelial cells, such as those of the bloodbrain barrier. Their sealing properties arise because they are zonular in nature, encircling the entire apicolateral margins of simple epithelial cells, and because there is direct contact between the plasma membranes of adjacent cells. Tight junctions are also important in maintaining the composition of the different membrane domains of epithelial cells, because they prevent diffusion of molecules within the outer leaflet of the plasma membrane, thereby restricting them to either the apical or the basolateral domain (14). This property is sometimes referred to as the "fence" function of tight junctions, and their occluding properties are described as a "gate" function. Gap junctions, so-called because they exhibit a regular plasma membrane separation of 2 nm, are punctate membrane sites that provide hydrophilic channels for direct cell-to-cell communication (15). These ubiquitous junctions are important in both excitable and nonexcitable tissues. In the former they facilitate direct transmission of electrical impulses, while in the latter they mediate a phenomenon called metabolic cooperation, and they may also transmit signaling molecules such as calcium and inositol phosphates between cells. Their function is essential in embryonic development (16) and in the normal functioning of adult tissues-for example, in coordination of the contraction of cardiac muscle and smooth muscle in the uterus during parturition (17, 18). Gap junctions appear to be widespread in the animal kingdom, being present even in primitive organisms such as Hydra. Intermediate junctions have also been clearly demonstrated in invertebrates (eg, in Drosophila). The occurrence of desmosomes and tight junctions in invertebrates is much less clear. Desmosome-like structures are clearly present in insects-for example, between the apposed epithelia of the upper and lower surface of the wing blade in Drosophila. However, these desmosome-like structures appear to be associated with microtubules rather than intermediate filaments, and their adhesion may be mediated by integrins rather than cadherins. Tight junctions are absent from insects, which instead possess septate junctions, structures that have a ladder-like ultrastructural appearance between adjacent plasma membranes and which, like tight junctions, appear to restrict paracellular permeability (19). Bryant (1994) In Molecular Mechanisms of Epithelial Cell Junctions: From Development to Disease (S. This implies that multiple lineages of cells, not necessarily distinct from each other, coexist in the culture (1). Serial subculture, while maintaining these parallel lineages, will tend to show convergence towards whatever common phenotype is best adapted to the culture conditions being employed, as the cell type with the greatest proliferative ability will predominate. The cell line may be finite and die out after a fixed number of population doublings, or become a continuous cell line (see Immortalization). Cell lines, particularly continuous cell lines, can become a valuable resource, particularly if preserved in liquid nitrogen after appropriate characterization and validation. Many continuous cell lines are currently available, with wide-ranging properties, including drug resistance markers, inducibility for specific enzymes, estrogen sensitivity, cornification, blood vessel formation, hemoglobin synthesis, myogenesis, and transfection susceptibility (Table 1). Finite cell lines with distinct phenotypic properties are also becoming available through the development of serum-free selective media (2-4). Effect of continued culture passage on cumulative cell number, assuming that no cells are discarded. The curve shows the initial decline due to selection, then exponential growth during the replicative phase, and then growth arrest and eventual deterioration following senescence, in a finite cell line, or continued proliferation, often at an enhanced rate, following transformation. Cell Bi 39, 29a Caco-2 Epithelial Human Adult Neoplastic Aneuploid Transports ions Fogh (19 colon and amino acids J. Origin of Cell Lines Regenerating tissues in vivo are made up of a small, self-repopulating, stem cell pool, an expandable pool of proliferating progenitor cells, and a nonproliferating differentiated cell pool. On demand, cells leave the stem cell pool and enter the progenitor compartment, where expansion is regulated to meet the current demand in the differentiated cell compartment. When cells enter the differentiated cell compartment, this may be an irreversible process, as seen with erythrocytes, keratinocytes, and neurons; or it may be reversible, as seen with hepatocytes, endothelial cells, and fibrocytes. Cell lines can be derived from any tissue with a proliferating compartment, or from cells that can re-enter a proliferating compartment. It is possible that cell lines will contain stem cells, but, except for hemopoietic cells (see Hematopoiesis), markers are unavailable to determine this. As many cell lines express lineage markers and can, under appropriate conditions, differentiate, it seems most likely that they are derived from the progenitor cells of the tissue.
Finally diabetes in dogs problems buy januvia pills in toronto, a particular amide nitrogen on an asparagine residue in certain phycocyanins and phycoerythrins diabetic diet vs regular diet cheap januvia 100 mg free shipping, involved in photosynthesis in cyanobacteria and red algae, may be methylated, possibly to enhance efficiency of energy transfer to the light-harvesting complex (2). Isomerized Aspartate Residues As proteins "age," L-aspartyl residues become isomerized to L-isoaspartyl residues or racemized to D-aspartyl residues. Then these residues may become methylated, and their spontaneous demethylation sometimes restores the original L-isomer. The succinimide intermediate can be generated by deamidation of an asparagine residue. Surette (1996) In Escherichia coli and Salmonella, Cellular and Molecular Biology, 2nd ed. In a mechanism first described by Wu and Santi (3) and later modified by Erlanson, et al. The proposed mechanism involves a direct transfer of the methyl group from AdoMet to the amino group, rather than through a covalent intermediate. M·HhaI shows significant distortion of the phosphate backbone of the strand in which the extrahelical cytosine is located, along with interdigitation of two amino acid residues from the recognition domain into the cavity opposite the orphaned guanine base. The overall structural similarity of C5-cytosine methyltransferases suggests that base flipping may be a common mechanism for this family of enzymes. The expelled adenine base, which is complementary to one of the bases in the thymine dimer, is trapped in a pocket on the surface of the enzyme (11). Although the expelled base in this structure is opposite the strand in which the thymine dimer resides, its exit from the helix renders the damaged bases accessible to the repair enzyme. The detailed mechanism of how the cytosine or adenine bases are extruded remains speculative. Aberrant methylation patterns in mammals promote tumorigenesis and lead to developmental abnormalities (12, 13). Since eukaryotic C5-methylcytosine methyltransferases have amino acid sequence similarities to their prokaryotic relatives, the results from studies of the bacterial enzymes will likely contribute to understanding their eukaryotic counterparts. The enzyme also repairs other alkyl guanines, however, such as O6ethylguanine and O6-butyl-guanine, albeit at a much lower efficiency. Enzymes from these sources exhibit considerable sequence homology and contain the active-site cysteine residue within the ProCys HisArgValsignature sequence. It performs three functions (2): (i) positive transcriptional regulator, (ii) phosphotriester methyltransferase, and (iii) O 6-methylguanine methyl transferase. Nakabeppu (1988) Regulation and expression of the adaptive response to alkylating agents. Methyltransferase Methyltransferases use S-adenosylmethionine (AdoMet) as a methyl donor to catalyze methylation of functional groups on amino acids, usually on their side chains (1). Many different amino acids in proteins are methylated by a host of specific enzymes (see Methylation, Protein). The methyl group donated from AdoMet is on a sulfonium ion (trivalent sulfur atom having a "formal" positive charge). Perhaps the most well characterized methyltransferases are the CheR methyltransferases in bacteria involved in Chemotaxis. These residues are located on two a-helical coiled coils within the cytoplasmic region of the receptors, known as methyl-accepting chemotaxis proteins (2). These methylated residues are located in different places in the receptors of Bacillus subtilis and Escherichia coli (3). Interestingly, in some instances the glutamate residue is produced from a genetically encoded glutamine (see Genetic Code) by the CheB methylesterase acting as a deamidase. This enzyme also hydrolyzes the methyl esters produced by the action of CheR (2). After autophosphorylation, the CheA kinase leads to increases in the concentrations of the phosphorylated forms of CheY and CheB. In other words, upon activation, CheA simultaneously causes an excitatory event (due to phosphorylated CheY) and sets in motion the adaptation process (due to the action of phosphorylated CheB). Other methyltransferases catalyzing methylesterifications have been identified and characterized. These include the enzyme that catalyzes the metabolically labile methylation of the carboxylterminal leucine residue of protein phosphatase 2A, an enzyme important in regulating cell metabolism (7). The methylation and demethylation represent a novel device for regulating the phosphatase itself. In bacteria, the methyltransferase that catalyzes methylation of isoaspartate residues, an isomer of aspartate that occurs when proteins age, has been identified and the gene disrupted.
Safe 100mg januvia. New genetic testing may better identify diabetes risk.
Origin of A Central Intron By the time the first chironomid globin genes were cloned in 1984 diabetes mellitus vs type 2 buy 100 mg januvia overnight delivery, the sequencing of vertebrate globin genes had become a cottage industry metabolic disease basal ganglia purchase januvia visa. In a prophetic para (14), Go analyzed X-ray diffraction data for a vertebrate globin and concluded that in addition to the outer introns, the ancestral globin gene had a third intron in the center of the gene such that the entire gene was separated into four exons of similar length, each coding for a separate structural domain. Unlike the conservation of location of the outer vertebrate globin gene introns, the location of central introns is not conserved across kingdoms or phyla, and none is at the position predicted by Go. Could they, like the outer introns in globin genes, still be descendants of an ancestral intron? Gilbert suggested that introns at nonconserved locations have moved by "intron sliding. A chironomid mitochondrial gene phylogeny groups flies with an intron at one location ("type 1") on the "pseudothummi" branch, and groups those with an intron at the other location ("type 2") on a separate "plumosus" evolutionary branch (8, 16, 17). The phylogeny supports the separate acquisition of an intron by the homologous genes after divergence of the two lineages. An intron once acquired by a single gene might spread to nearby related genes by gene conversion (8); see. The phylogenetic study summarized in Figure 2 traces the evolutionary history of the two clusters, showing the seven genes that are clearly homologous, and a series of gene duplications and deletions necessary to explain the current sequence and organization of the clusters. Pairwise comparisons show that at the nucleotide level, homologous globin genes or logical gene pairs (ie, genes of indeterminate orthology, but sharing immediate common ancestry) in the two species share from 96. In three cases, homologous genes in each species code for identical polypeptides, despite several millions of years of species divergence. Since the recombination point is in fact external to the transcribed region of the gene, 7B9/5 must simply be another ct-7B5 gene or allele. The recombined region (without ct-7B10) may be an independent gene locus or an allelic haplotype. The 7B gene cluster seems to have undergone periodic expansion and contraction (gene duplication, deletion), while maintaining the ability to encode identical or nearly identical globins for millions of years. The best explanation for this phenomenon is that the few differences that accumulate between 7B genes are the result of random drift (neutral evolution), but that the cluster itself has experienced positive selection of a high number of copies of globin genes as a means to ensure the synthesis of large amounts of hemoglobin. Figure 1 summarizes evolution of these genes after acquisition and spread of a "type 1" intron in one of the clusters. Figure 1 reflects more recent phylogenetic evidence that some or all of the genes in these clusters arose early in the genus, and that the C. Of special note, the recent inactivation of a different (non homologous) gene in each cluster suggests that not all globin genes in a cluster are indispensable, even after 60 million years of evolution. Again, this event must have occurred very recently, because the gene retains all other attributes of a viable, transcribable gene. If the greater diversity of the tentans and thummi genes cannot be completely explained as the positive adaptation of structurally diverse hemoglobins, then their maintenance, like that of the large number of 7B genes in thummi and piger, must be the result of positive gene copy number selection favoring high levels of hemoglobin synthesis. In sum, the evolution of Chironomus globin genes spans more than 250 million years, in which time individual sequences within and across species have accumulated many amino acid substitutions. Some especially diverged hemoglobins may have evolved to serve unique functions, for example in environments that undergo cyclic changes in oxygenation. On the other hand, differences between many of the globin genes may be neutral, selection favoring the proliferation of a large family of genes encoding proteins of physiologically similar function. Kimura (21) suggested that duplicated genes accumulating neutral change are preadapted, later becoming substrates for positive adaptation after speciation. A consequence of high gene copy number selection is that some of the many functionally redundant globin genes can serve as raw materials for Darwinian selection, which could explain the evolution and spread of more than 5000 species of Chironomous to diverse habitats. Beermann (1963) Cytological aspects of information transfer in cellular differentiation. Wieslander (1992) Secretory proteins of Chironomous salivary glands: structural motifs and assembly characteristics of a novel biopolymer. Vinogradov (1998) An electrospray ionization mass spectroscopic study of the extracellular hemoglobins from Chironomus thummi thummi. Chloramphenicol Chloramphenicol is a bacteriostatic agent that inhibits the growth of many species of Gram-positive and Gram-negative bacteria; it was the first broad-spectrum antibiotic to be used clinically. Originally obtained from cultures of the soil bacterium Streptomyces venezuelae, chloramphenicol inhibits bacterial protein biosynthesis by interfering with the intrinsic catalytic activity of the peptidyl-transferase of the ribosome during the elongation phase of translation. Nevertheless, the clinical application of this drug for the treatment of systemic infections has been severely curtailed, primarily because of the haematotoxicity associated with its use. Chloramphenicol continues to be used topically, particularly in the treatment of eye infections.
For small proteins (~25kDa) these fluctuations are on the microsecond timescale diabetes symptoms feet tingling discount januvia 100 mg overnight delivery, while for cells they are in milliseconds treatment diabetes before insulin best 100mg januvia. The fluctuations are related to the Brownian motion of the particles giving rise to density fluctuations caused by variations in the number of molecules in the scattering volume and random agglomerations. This measurement in the time domain is related to the spectral density of the fluctuations in the frequency domain by a Fourier transformation. In practice, diffusion coefficients are determined using an autocorrelation function that is measured by accumulating the product of the number of photons arriving at the detector from successive time intervals. The intensity autocorrelation function, G2(t), is obtained by storing the average products ItIt+t, where t is an incremented time delay, in successive channels to yield (1) For a solution of macromolecules, assuming a Gaussian distribution of fluctuations, G2(t) is related to the scattered electric field autocorrelation function G1(t) by (2) For translation diffusion of monodisperse particles that are small with respect to the incident wavelength, l: (3) where A and B are constants that depend upon experiment geometry, and (4) D is the translational diffusion coefficient, and G is the reciprocal of the characteristic decay time, (5) where O is the scattering angle. For a continuous polydisperse system, equation (4) is integrated over all sizes, and hence G values, to give (6) where G(G) is a distribution function that can be evaluated. Rotational diffusion and internal dynamics can also influence the autocorrelation functions measured in a dynamic light scattering experiments. The internal dynamics of a macromolecule in solution become important only if the amplitude of the motions is not very much smaller than the wavelength of the light. For large motions, different parts of the sample molecule will scatter out of phase, and the internal dynamics will contribute one or more relaxation times that will contribute to the decay of the autocorrelation function in a single or multiple-exponential fashion. The pharmacological effects of drugs can depend upon their behavior in solution and the characteristics of aggregates they may form (2). Lipids can be used to "solubilize" drugs that may be insoluble in aqueous media, and hence enhance their transport and efficacy. Dynamic light scattering has been used to evaluate aggregation behavior of antidepressants (3), critical micelle concentrations, and sizes of micelles designed to encapsulate the drug Indomethecin (4), as well as to evaluate how the opiate drug loperamide alters the temperature-induced phase transition of phosphatidylcholine vesicles (5). Dynamic light scattering has also been used to monitor the assembly of enveloped viruses. Dynamic light scattering has recently become a common tool for assessing the crystallizability of macromolecules and macromolecular assemblies (7, 8). Because modern molecular biology techniques have provided sufficient amounts of pure materials, dynamic light scattering can be used automatically to "screen" large numbers of crystallization conditions to determine diffusion constants and hence evaluate possible aggregation states that inhibit crystallization. Pecora (1976) Dynamic Light Scattering with Applications to Chemistry, Biology and Physics, Wiley, New York. Pecora (1985) Dynamic Light Scattering: Applications of Photo Correlation Spectroscopy, Plenum Press, New York. The dyneins are classified as either cytoplasmic or flagellar (also known as axonemal), and the flagellar dyneins are further divided into outer and inner arm dyneins. The first dyneins to be identified were the flagellar dyneins, which are the arms that project from the A microtubules of ciliary and flagellar axonemes. Flagellar dyneins generate the sliding force between microtubules that is the basis for the propulsive bending of flagella (and cilia) that moves cells through fluids. Cytoplasmic dyneins move membranous organelles and kinetochores along microtubules and assist in assembling the mitotic spindle. Each dynein is a large molecular complex, composed of one or more large polypeptide chains of ~500kDa, known as heavy chains, and various accessory intermediate chains (50 to 140 kDa) and light chains (8 to 45 kDa). An ~350kDa portion of each heavy chain forms a large globular head domain, and the rest of the heavy chain extends from the head as a thin flexible stalk. Two or three dynein heavy chains are often connected by their thin stalks to a common base. The base is made up of the intermediate and light chains, which are important for binding dynein to the organelles that it transports (its cargo). To date, only two uniquely cytoplasmic dynein heavy-chain genes have been identified. The heavy chain of this dynein is encoded by one gene, but multiple genes have been identified for the intermediate and light intermediate chains and for the 14-kDa light chain. The heavy chain of the minor cytoplasmic dynein is encoded by a unique gene, but the intermediate or light chains, with which it is presumed to be associated, have not yet been identified. Unlike cytoplasmic dynein, flagellar outer arm dyneins have two or three different heavy chains. Inner arm dyneins are either heterodimers or monomers of heavy chains and have various accessory polypeptides. Structural analysis of the heavy-chain motor domain indicates that the head is made up of seven lobes arranged in a ring surrounding a central cavity (1). Dyneins can be attached to glass coverslips via their bases and their heads will bind microtubules.