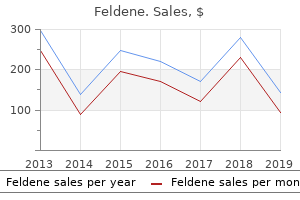
Feldene
"Purchase feldene 20 mg overnight delivery, extensive arthritis in neck".
By: E. Navaras, M.A., M.D.
Deputy Director, Mayo Clinic Alix School of Medicine
Exparel (bupivacaine liposome injectable suspension) rheumatoid arthritis research purchase 20mg feldene with amex, approved in 2011 for single use injection into a surgical site to produce postsurgical analgesia (pain relief) arthritis in your knee purchase feldene with american express. It was approved in 2018 for use as a nerve block to help adult patients with pain management for 48 hours after shoulder surgery. This approval helps to fill a need for additional nonaddictive pain management tools by providing a new option for certain patients. Invokana (canagliflozin), originally approved in 2013 as an adjunct to diet and exercise to improve glycemic (blood sugar) control in adults with type 2 diabetes mellitus. It was approved in 2018 in the same combination and for the same condition as its 2017 approval but also for women who have not been through menopause. Anaplastic is a term used to describe cancer cells that divide rapidly and have little or no resemblance to normal cells. This approval demonstrates that targeting the same molecular pathway in diverse diseases is an effective way to expedite the development of treatments that may help more patients. Mekinist was also originally approved in 2013 for treatment of certain patients with melanoma (skin cancer). Separately, Tafinlar was originally approved in 2013 to treat certain patients with melanoma. It was approved in 2018 to also treat certain patients who are newly-diagnosed with the condition. This drug received accelerated approval in 2016 and then regular approval in June 2018 for treating certain patients with chronic lymphocytic leukemia and small lymphocytic leukemia. Xeljanz (tofacitinib), first approved in 2012 to treat certain patients with rheumatoid arthritis. It was approved in 2018 to treat adults with moderately to severely active ulcerative colitis, a chronic, inflammatory bowel disease affecting the colon. Latuda (lurasidone), originally approved in 2010 to treat patients with schizophrenia - and subsequently approved in 2013 with a new indication to treat adult patients with depressive episodes associated with bipolar I disorder (bipolar depression). In 2018, it was approved to treat children and adolescents with major depressive episodes with bipolar I disorder. Tasigna (nilotinib) originally approved in 2007 to treat adults with certain forms of chronic myeloid leukemia in patients resistant to prior therapy with other treatments. It was approved in 2018 to treat pediatric patients one year of age or older with the same conditions. Biological products are highly complex, and often used to treat patients with serious and life-threatening conditions. Myelosupression is reduced bone marrow activity leading to low production of red blood cells, white blood cells, and platelets. It is approved to decrease the chance of infection for certain patients receiving myelosuppressive anti-cancer drugs (those that reduce bone marrow production of platelets, and red and white blood cells); reducing the time to restore white blood cells and recovery from fever, following certain chemotherapy treatment of patients with acute myeloid leukemia; reducing the duration of low white blood cell count episodes and their adverse effects, such as fever and infections, in certain patients undergoing chemotherapy followed by bone marrow transplantation; to enhance the process of leukapheresis (a laboratory procedure in which white blood cells are separated from a sample of blood); and chronic administration to reduce the incidence and duration of the adverse effects of low white blood cell count. Retacrit is also approved for use before and after surgery to reduce the chance that red blood cell transfusions will be needed because of blood loss during surgery. This includes at least one biosimilar for each of these top selling biological drugs in the United States: Humira, Rituxan, Enbrel, Herceptin, Avastin, Remicade, and Neulasta. Two biological reference products (Humira and Remicade) now have three biosimilars each, three reference products (Herceptin, Neulasta, and Neupogen) have two biosimilars each, and four reference products (Avastin, Enbrel, Epogen/Procrit, and Rituxan) currently each have one biosimilar. An increase in market competition may lead to significantly reduced costs for both patients and our healthcare system. New formulations of already-approved drugs can offer significant advances in therapy. After a loading regimen (multiple doses to reach the intended blood level), the drug is taken once weekly. Aristada Initio (aripiprazole lauroxil), a new formulation of the drug, Aristada (aripiprazole), approved in 2015 to treat patients with schizophrenia. This new formulation is to be used as an intramuscular injection in combination with oral aripiprazole to initiate therapy. Prior to approval of Aristada Initio, dosing for aripiprazole needed to be gradual, starting at a low dose and gradually increasing to an effective dose. This new formulation enables immediate dosing to therapeutic levels - an important feature to help treat symptoms of schizophrenia as soon as possible. Atropine autoinjector, for emergency use in the initial treatment of muscarinic symptoms of poisoning by certain insecticides, or nerve agents, including those that can be used in bioterrorism attacks.
In this case patients are randomized to active tablets plus placebo infusion or to active infusion plus placebo tablets arthritis eating disorders discount feldene 20 mg otc. To place reliance on a negative result arthritis medication for dogs buy discount feldene on line, the statistical power of the study should be at least 0. It is possible to calculate the number of patients required to establish a given difference between treatments at a specified level of statistical confidence. For a continuous variable, one needs an estimate of the mean and standard deviation which one would expect in the control group. This is usually available from historical data, but a pilot study may be necessary. For example, if in an antihypertensive study comparing two treatments administered for three months only the data from those who completed three months of therapy with treatment X or Y are analysed, this may suggest that both treatments were equally effective. However, if 50% of the patients on treatment X withdrew after one week because of lack of efficacy, that conclusion is erroneous. Conversely, if patients are withdrawn after randomization but before dosing, adverse events cannot be attributed to the drug. It is therefore very important to prespecify the primary trial end-point and secondary end-points that will be analysed. Statistical corrections can be applied to allow for the number of comparisons made. One must also consider the clinical importance of any statistically significant result. For example, a drug may cause a statistically significant decrease in blood pressure in a study, but if it is only 0. This process is repeated until some predefined end-point such as a particular plasma concentration, a pharmacodynamic effect or maximum tolerated dose is reached. Data from the single-dose study will determine appropriate doses and dose intervals for subsequent multiple-dose studies. If the drug is administered by mouth, a food interaction study should be conducted before multiple-dose studies. Key points Phase I studies: initial exposure of humans to investigational drug; assessment of tolerance, pharmacokinetics and pharmacodynamics in healthy subjects or patients; usually healthy male volunteers; usually single site; 40100 subjects in total. If it is ethical and practicable, a double-blind design is used, employing either a placebo control or a standard reference drug therapy as control. These are the first studies in the target population, and it is possible that drug effects, including adverse drug reactions and pharmacokinetics, may be different to those observed in the healthy subjects. The first dose to be administered to humans is usually a fraction of the dose that produced any effect in the most sensitive animal species tested. Subjective adverse events, clinical signs, haematology, biochemistry, urinalysis and electrocardiography are used to assess tolerability. Depending on the preclinical data, further, more specific evaluations may be appropriate. The studies are placebo controlled to reduce the influence of environment and normal variability. Yes Are the benefits statistically significant over existing therapy/placebos in well-designed clinical trials? Yes No No Patient groups who respond more or less well may be identified, patient exposure (both numbers and duration of therapy) is increased, and less common type B (see Chapter 12) adverse reactions may be identified. During this period, the manufacturers will be setting up plant for large-scale manufacture and undertaking further pharmaceutical studies on drug formulation, bioavailability and stability. Marketing approval may be general or granted subject to certain limitations which may include restriction to hospital practice only, restriction in indications for use, or a requirement to monitor some particular action or organ function in a specified number of patients. In practice, the essential point is that clinically untoward consequences should not ensue if one preparation is substituted for the other. Interestingly, studies have shown that patients taking part in clinical trials often have better health outcomes than those not involved in a trial. The goal is to facilitate the early introduction of valuable new therapies, while at the same time maximizing patient protection.
Nonresident Wholesaler or Nonresident Third-Party Logistics Provider; Surety Bond Requirements 4163 arthritis in dogs help buy feldene no prescription. Sale or Transfer of Dangerous Drug or Device into State: Furnishing Records to Authorized Officer on Demand; Citation for Non-compliance 4166 arthritis pain disability purchase feldene with visa. Shipping of Dangerous Drugs or Devices - Wholesaler or Distributor Liable for Security and Integrity until Delivery 12 Article 11. Wholesaler: Bar on Obtaining Dangerous Drugs or Devices It Cannot Maintain on Licensed Premises 4168. Surplus Medication Collection and Distribution Intermediary; License Required Article 11. Veterinarian in Teaching Hospital May Dispense and Administer Dangerous Drugs and Devices; Requirements 4171. Exceptions to Section 4170: Samples; Clinics; Veterinarians; Narcotic Treatment Programs; Certain Cancer Medications 4172. Independent Clinics Sharing Office Space Licensing; Separate Boards, Drug Stocks, Records; Reporting Losses; Application Fee; Ownership Change; Review by 13 Article 13. Clinic Defined; License Required; Purchase of Drugs at Wholesale: Drug Distribution Service of a Clinic; Information Reported to the Board 4191. Compliance with Department of Health Services Requirements; Who May Dispense Drugs 4192. Clinic Not Eligible for Professional Dispensing Fee; Ban on Offering Drugs for Sale 14 Article 14. License Required: Temporary License on Transfer of Ownership; Board Approval of Designated Representative-in-Charge 4197. Written Policies and Procedures Required: Contents; Training of Personnel; Quality Assurance; Consulting Pharmacist 4199. Pharmacist License Requirements: Age; Education; Experience; Examination; Proof of Qualifications; Fees 4200. California Practice Standards and Jurisprudence Examination for Pharmacist; Required Inclusions 4200. Retired Licensee: Eligibility; Bar on Practice; Requirement for Restoration to Active Status 4201. Application Form: Required Information; Authority Granted by License; Reporting Changes in Beneficial Ownership 15 Article 16. Applications Pharmacy Technician: License Requirements for Education, Experience; Board Regulations; Criminal Background Check; Discipline 4202. Sale or Dispensing of Hypodermic Syringes and Needles: When Separate License Required; Form and Content of Application; Renewability; Discipline 4207. Advanced Practice Pharmacist; License Renewal; Placed on Inactive Status by Board 4202. Requirements for Renewal of Pharmacist License: Clock Hours; Exemption for New Licensee 4232. Revocation and Suspension: Authority; Conditions; Issuance of Probationary License; Application of Administrative Procedure Act; Judicial Review 4300. Pharmacist License; Out-of-State Suspension or Revocation to Apply to California License 4302. Discipline of Corporate Licensee for Conduct of Officer, Director, Shareholder 4303. Outsourcing Facility: License Canceled, Revoked or Suspended by Operation of Law 4304. Disciplinary Grounds: Failure of Pharmacy, Pharmacist to Notify Board of Termination of Pharmacist-in-Charge; Continuing to Operate Without Pharmacist 4305. Disciplinary Grounds: Failure of Wholesaler, Veterinary Food-Animal Drug Retailer or Third-Party Logistics Provider to Notify Board of Termination of Designated Representative-in-Charge or Responsible Manager; Continuing to Operate Without a Designated Representative-in-Charge or Responsible Manager 4306. Prohibited Association: Notification of Affected Licensees Known to Board Petition for Reinstatement, etc.
The vast majority of microbes are harmless to us arthritis leg pain buy discount feldene line, and many play essential roles in plant arthritis of the ankle buy discount feldene online, animal and human health. Others, however, are either obligate or facultative pathogens exerting a spectrum of deleterious effects on their hosts. Infectious diseases have historically represented the most common cause of death in humans until recently, exceeding by far the toll taken by wars or famines. From the dawn of humanity and throughout history, infectious diseases have shaped human evolution, demography, migrations and history. A pathogen is defined as an organism causing disease to its host, with the severity of the disease symptoms referred to as virulence. Pathogens are taxonomically widely diverse and comprise viruses and bacteria as well as unicellular and multicellular eukaryotes. Every living organism is affected by pathogens, including bacteria, which are targeted by specialized viruses called phages. The number of viruses and bacteria on earth is staggering and they occupy essentially every environment. A liter of surface seawater typically contains in excess of ten billion bacteria and 100 billion viruses. The number of viruses on Earth is estimated to be around 1031, which corresponds to roughly ten billion times the number of stars in the universe [1]. An average human is made up of about 30 trillion cells but carries a similar number of bacteria, mostly in the gut [2]. The vast majority of viruses and bacteria we are exposed to have no negative effect and some can even * Correspondence: f. Pathogens can be divided into two main categories, namely facultative and obligate pathogens, reflecting how intimately their life cycle is tied to their host. Facultative pathogens are organisms for which the host is only one of the niches they can exploit to reproduce. Facultative pathogens are primarily environmental bacteria and fungi that can occasionally cause infection. They include many of the most problematic hospital-acquired bacteria involved in the antimicrobial resistance pandemic. A distinction is sometimes made between facultative and accidental pathogens, with the latter representing those which only occasionally infect weakened or immunocompromised hosts. All viruses are obligate pathogens as they are dependent on the cellular machinery of their host for their reproduction. Obligate pathogens are found among bacteria, including the agents of tuberculosis and syphilis, as well as protozoans (such as those causing malaria) and macroparasites. Some obligate pathogens require multiple different hosts to fulfil their life cycle. The definite host, which supports the adult form of the pathogen, is often a vertebrate and the intermediate host (referred to as a vector) is generally an arthropod or a mollusc. This alternation of vertebrate and invertebrate hosts is found in viruses (for example the Zika virus), bacteria (for example Lyme disease) and protozoa (malaria). Trematodes (parasitic flatworms) go even further and some exhibit among the © Balloux et al. Digenetic trematodes have a basic three-host life cycle, and for some species a fourhost life cycle. For instance, Halipegus occidualis sequentially has to infect a freshwater snail, an ostracod, a dragonfly nymph and ends its cycle after the dragonfly is eaten by the green frog Rana clamitans, where it resides under its tongue [3]. Some pathogens are limited to infecting a single host species, whereas others can infect a multitude of host species. For example, leprosy in humans is caused by two related intracellular bacteria Mycobacterium leprae and Mycobacterium lepromatosis, which are essentially restricted in the wild to humans, as well as armadillos in the Americas and red squirrels in Scotland [4]. Conversely, Yersinia pestis, another intracellular obligate bacterium and the agent of plague, has a natural life cycle involving alternating infections of rodents and fleas, but can infect essentially any mammalian host.
Order feldene 20mg visa. Paddison Podcast #18 - Dr Michael Greger on Rheumatoid Arthritis and Optimal Health.