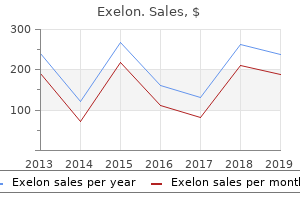
Exelon
"Exelon 3mg on-line, medications similar to adderall".
By: T. Bernado, M.A., M.D., M.P.H.
Assistant Professor, University of Michigan Medical School
Emerging Minimally Invasive Treatment Options for Male Lower Urinary Tract Symptoms medicine 2015 purchase exelon with paypal. Prostate artery embolisation for benign prostatic hyperplasia: a systematic review and meta-analysis symptoms xanax treats buy discount exelon 3 mg. Minimally invasive prostatic urethral lift: surgical technique and multinational experience. Benign prostatic hyperplasia and new treatment options a critical appraisal of the UroLift system. Three-Year Outcomes of the Prospective, Randomized Controlled Rezm System Study: Convective Radiofrequency Thermal Therapy for Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia. Minimally Invasive Prostate Convective Water Vapor Energy Ablation: A Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Erectile and Ejaculatory Function Preserved With Convective Water Vapor Energy Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Randomized Controlled Study. Rezm Water Vapor Thermal Therapy for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: 4-Year Results From Randomized Controlled Study. Is Sexual Function Better Preserved After Water Vapor Thermal Therapy or Medical Therapy for Lower Urinary Tract Symptoms due to Benign Prostatic Hyperplasia? Society of Interventional Radiology position statement: prostate artery embolization for treatment of benign disease of the prostate. Transurethral resection versus minimally invasive treatments of benign prostatic hyperplasia: results of treatments. Rezm System Water Vapor Treatment for Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia: Validation of Convective Thermal Energy Transfer and Characterization With Magnetic Resonance Imaging and 3-Dimensional Renderings. Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: protocol for a non-inferiority clinical trial. Prostatic urethral temporary implant insertion for lower urinary tract symptoms caused by benign prostatic hyperplasia. Transurethral water jet ablation for lower urinary tract symptoms caused by benign prostatic hyperplasia. UroLift for Treating Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Prostate artery embolisation for lower urinary tract symptoms caused by benign prostate hyperplasia. Rezum for treating lower urinary tract symptoms secondary to benign prostatic hyperplasia. Transurethral water vapour ablation for lower urinary tract symptoms caused by benign prostatic hyperplasia. Energy delivery systems for treatment of benign prostatic hyperplasia: an evidence-based analysis. Safety and efficacy of transurethral needle ablation of the prostate for symptomatic outlet obstruction. Prostatic urethral lift improves urinary symptoms and flow while preserving sexual function for men with benign prostatic hyperplasia: a systematic review and metaanalysis. Randomised clinical trial of prostatic artery embolisation versus a sham procedure for benign prostatic hyperplasia. Symptom relief and anejaculation after aquablation or transurethral resection of the prostate: subgroup analysis from a blinded randomized trial. Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clinical evaluation of embolization of the superior vesical prostatic artery for treatment of benign prostatic hyperplasia: a single-center retrospective study. Microwave thermotherapy for benign prostatic hyperplasia with the Dornier Urowave: results of a randomized, double-blind, multicenter, sham-controlled trial. Prostatic Urethral Lift: A Unique Minimally Invasive Surgical Treatment of Male Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Convective Thermal Therapy: Durable 2-Year Results of Randomized Controlled and Prospective Crossover Studies for Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia.
Strong recommendation treatment scabies exelon 4.5 mg sale, moderate quality of evidence Summary of Evidence A meta-analysis by Green et al symptoms type 1 diabetes buy exelon 3 mg on-line. The effectiveness of this regimen needs to be confirmed in a randomized controlled trial. As monotherapies, one randomized study compared the use of ciprofloxacin 250 mg daily vs. Third, in a country where tuberculosis is highly endemic, it is prudent to reserve ciprofloxacin as a second-line drug for multidrug-resistant tuberculosis. Different trials have reported antimicrobial prophylaxis durations ranging from four21,22 to six20,23 to eight-and-a-half months. Newer interventions, such as perioperative intravesical application of antibiotic solution after renal transplantation, have been looked into in one study. More studies are needed before a recommendation regarding this intervention can be made. Management of urinary tract infections and lymphocele in renal transplant recipients. Late urinary tract infection after transplantation: prevalence, predisposition and morbidity. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Frequency of urinary tract infection in renal transplant recipients and effect on graft function. Late recurrent urinary tract infections may produce renal allograft scarring even in the absence of symptoms or vesicoureteric reflux. Antibiotic prophylaxis for urinary tract infections in renal transplant recipients: a systematic review and meta-analysis. A prospective, randomized, double-blind study of trimethoprim-sulfamethoxazole for prophylaxis of infection in renal transplantation: clinical efficacy, absorption of trimethoprim-sulfamethoxazole, effects on the microflora, and the costbenefit of prophylaxis. Efficacy of high-dose trimethoprimsulfamethoxazol prophylaxis on early urinary tract infection after renal transplantation. Effect of ciprofloxacin combined with sulfamethoxazoletrimethoprim prophylaxis on the incidence of urinary tract infections after kidney transplantation. Trimethoprim-sulfamethoxazole compared with ciproloxacin for the prevention of urinary tract infection in renal transplant recipients. A controlled study of trimethoprimsulfamethoxazole prophylaxis of urinary tract infection in renal transplant recipients. Use of ciprofloxacin as a prophylactic agent in urinary tract infections in renal transplant recipients. Is perioperative intravesically applied antibiotic solution effective in the prophylaxis of urinary tract infections after renal transplantation? Should patients who will undergo urologic procedures receive perioperative prophylactic antibiotics? Selected patients should receive perioperative prophylactic antibiotics to prevent the occurrence of healthcare-associated infections arising from diagnostic and therapeutic urologic procedures. Strong recommendation, low quality of evidence Summary of Evidence the goal of perioperative antibiotic prophylaxis is to prevent healthcareassociated infections arising from diagnostic and therapeutic procedures. Perioperative antibiotic prophylaxis has been controversial especially with the lack of good studies to support its use. Three systematic reviews have consistently reported the benefit of prophylaxis-the short course (<72 hours) regimen, in particular-in decreasing the incidence of postoperative bacteriuria (from 26% to 9%) and other related complications. The presence of these factors is a reason to administer antibiotic prophylaxis or prolong its duration in an otherwise low-risk urological procedure due to the vulnerability of this set of patients. What is the approach to giving perioperative antibiotic prophylaxis in a patient who will undergo a urologic procedure? The decision on whether to continue or shift antibiotics and the duration after the procedure will depend on the best clinical judgment of the physician. Generally accepted risk factors for infectious complication General risk factors Special risk factors associated with an increased bacterial load Older age Long preoperative hospital stay or recent hospitalization, complicated Deficient nutritional status History of recurrent urogenital infections Impaired immune response Surgery involving bowel segment Diabetes mellitus Colonization with microorganisms Smoking Long-term drainage Extreme weight Urinary obstruction Coexisting infection at a remote site Urinary stone Lack of control of risk factors Adapted from Grabe et al.
Arm 3 medications like zovirax and valtrex purchase exelon on line, and Arm 2 and Arm 3) after adjusting for the baseline and stratification factors with a significance level of 0 medicine werx safe 3 mg exelon. All results from the imputed analysis using the multiple imputation will be compared to the complete case analysis results to assess any potential biases. We will conduct a sensitivity analysis using various assumptions on the missing data to determine what impact missing data and imputation methods have on the study conclusions. Imputation methods when prescribed by validated instrument developers will be employed first. Group Sequential Testing for Early Termination and Reporting of Efficacy and Futility (1/8/09) A group sequential test with three planned interim analyses and a final analysis will be performed. The interim analysis will be carried out when the cumulative accrual (patients whose follow-up is at least 5 years from the randomization date) are met. For each interim analysis, one efficacy and two futility tests will be carried out. For the futility testing boundary, we will use a less aggressive boundary, Rule C in Freidlin and Korn. Before making such a recommendation, the accrual rate, treatment compliance, safety of the treatments, and the importance of the study are taken into consideration along with the p-value. Interim Report to Monitor the Study Progress Interim reports with descriptive statistics will be prepared twice per year until the initial paper reporting the treatment results has been submitted. In general, the interim reports will contain information about the patient accrual rate with a projected completion date for the accrual phase, compliance rate of treatment delivery with the distributions of important prognostic baseline variables, and the frequencies and severity of the adverse event by treatment arm. The interim reports will not contain the results of the treatment comparisons with respect to the primary endpoint and secondary endpoints. Reporting the Initial Treatment Analysis (1/8/09) the analysis reporting the treatment results will be carried out after the criteria for early stopping/reporting are met. Three interim comparisons and one final analysis will be performed for efficacy and futility of the experimental treatment will be carried out as described in section 13. It will include tabulation of all cases entered and those excluded from the analyses; the distribution of the important prognostic baseline variables; safety treatments; treatment compliance; and observed results with respect to the primary and secondary endpoints will be shown. All eligible patients randomized will be included in the comparison and will be grouped by assigned treatment in the analysis (intent-to-treat analysis). Adjuvant and salvage radiation therapy after radical prostatectomy for adenocarcinoma of the prostate. Positive resection margin and/or pathologic T3 adenocarcinoma of prostate with undetectable postoperative prostate-specific antigen after radical prostatectomy: To irradiate or not? Salvage radiotherapy after radical prostatectomy for prostate adenocarcinoma: Analysis of efficacy and prognostic factors. Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Tolerance and early outcome results of postprostatectomy three-dimensional conformal radiotherapy. Effect of radiation therapy on detectable serum prostate specific antigen levels following radical prostatectomy: Early versus delayed treatment. Impact of postprostatectomy prostate-specific antigen nadir on outcomes following salvage radiotherapy. Salvage radiotherapy for biochemical failure of radical prostatectomy: A single-institution experience. Radiotherapy for men with isolated increase in serum prostate specific antigen after radical prostatectomy. The efficacy of postprostatectomy radiotherapy in patients with an isolated elevation of serum prostate-specific antigen. Salvage radiotherapy for biochemical recurrence after radical prostatectomy: a study of 62 patients. Adjuvant radiotherapy for pathologically advanced prostate cancer: A randomized clinical trial. Effect of combined transient androgen deprivation and irradiation following radical prostatectomy for prostatic cancer. Neoadjuvant hormone therapy before salvage radiotherapy for an increasing post-radical prostatectomy serum prostate specific antigen level.
Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies symptoms 4 dpo cheap 4.5mg exelon fast delivery. In the absence of a controlling federal or state coverage mandate treatment 7 february order 4.5mg exelon otc, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. Coverage Policies relate exclusively to the administration of health benefit plans. Coverage Policies are not recommendations for treatment and should never be used as treatment guidelines. In certain markets, delegated vendor guidelines may be used to support medical necessity and other coverage determinations. The most frequent indications for surgical management are moderate-tosevere voiding symptoms that are refractory to medical management. However, several other minimally invasive surgical procedures and therapies have been widely used and are supported by relevant professional societies. Generally, data in the published, peer-reviewed literature demonstrate improved outcomes and support the safety and effectiveness of these other established therapies. The delivery device contains a preloaded implant that deploys, self-adjusts, tensions, and trims a permanent tensioning suture. The suture runs from the urethra to the outer prostatic capsule and serves to compress the lateral lobe of the prostate. Four to 5 implants are typically inserted, but this varies with the size and shape of the prostate. The UroLift may be used to treat prostate glands measuring <80 milliliters (mL) in size in the United States. The UroLift System is generally implanted by an urologist in an outpatient or inpatient setting. The transurethral procedure to insert the UroLift is performed with the use of local or general anesthesia and oral sedation. The median lobe clinical study was the prospective Median Lobe Prostatic UroLift System Procedure (MedLift) study. In addition, literature data and medical opinion support lowering the age indication from 50 years old to 45 years old since Page 3 of 28 Medical Coverage Policy: 0159 there is no clinical difference between the two patient populations. As such, the UroLift System is substantially equivalent to the UroLift System cleared in K133281 and K172359. Minor modifications were made to the device including the delivery handle and the implant cartridge that do not affect the overall safety and effectiveness of the UroLift procedure. Evidence in the published, peer-reviewed scientific literature consists of randomized controlled trials and smaller prospective, retrospective, and case series studies. The guidance stated that surgical options should be considered after the failure of conservative management and medication. The Committee recognized that the duration of symptom relief after using the UroLift system is uncertain. It concluded that it is similar in the medium term (up to 3 years) to the comparators but that further evidence on durability and the need for subsequent procedures would be useful. The UroLift system should be considered as an alternative to current surgical procedures for use in a day-case setting in men with lower urinary tract symptoms of benign prostatic hyperplasia who are aged 50 years and older and who have a prostate of less than 100 ml without an obstructing middle lobe. The Rezm System is also indicated for treatment of prostate with hyperplasia of the central zone and/or median lobe. The device Page 5 of 28 Medical Coverage Policy: 0159 converts water into vapor outside of the body and the vapor is delivered to the prostate tissue via a needle within the sterile Delivery Device. The vapor ablates the targeted tissue within the prostate via thermal ablation as energy is transferred from the vapor to the prostate tissue. Literature Review: Although there is a paucity of data in the peer-reviewed scientific literature comparing water vapor thermal therapy. Treatment with the Rezm System is generally safe and not associated with loss of sexual function (Dixon, et al. The recommendations were based on results of the randomized controlled trial conducted by McVary et al. Ethanol injection is performed using dehydrated ethanol injected with a flexible injection needle through the side channel of a cystoscope and into the targeted tissue.
Over the past decade conventional medicine order exelon visa, several types of rapid test kits have been developed for use in the field acute treatment purchase exelon with amex. While their reliability in determining the amount of iodine present has been questioned (91), their use as an effective tool for quality control and assurance, and hence for advocacy, remains valuable. The total prevalence of goitre has been used for much of the recent past to assess and monitor iodine deficiency. This is especially so in those most vulnerable to the consequences of the deficiency: pregnant and lactating women and young infants. In many countries iodine deficiency is now under control and it is anticipated this progress will continue. These criteria include indicators related to salt iodization, urinary iodine status and to the programme itself. As noted above, the control of iodine deficiency is a continuous process; it cannot be interrupted without the risk of re-emergence of iodine deficiency and its resulting disorders. Iodine deficiency in Europe and its control: current status, progress and recent trends 3. While writing this review, national experts from each of the 40 countries were contacted and asked to provide their most current data on the subject. Surveys are considered as national level when they are carried out on a nationally representative sample of the population, or as subnational level when they are carried out on a sample representative of a given administrative level: region, state, province, district or local. Whenever available, data from the most recent national survey were used in preference to subnational surveys. When two or more subnational surveys of the same subnational level had been carried out in different locations of the country during the analysis period, the survey results were pooled into a single summary measure, using a weighted sample size for each survey. When data for this age group were not available, data of the next closest age group were used in the following order of priority: data from the children closest to school age, adults, the general population, preschool-age children, other population groups. The method used, including reference values applied, was carefully quoted for each survey and referenced. Serum thyroglobulin was used as a particularly sensitive marker of the degree of stimulation of the thyroid gland. As these data have come from national authorities and hence may have been collected from different formats, less rigorous criteria were used for their inclusion. In this case the data most similar to that in the tables were used and footnotes included. National data were available from 18 countries; 14 countries supplied subnational data (Figure 3. It should be noted that in some cases subnational data were representative of only selected areas within a country. These countries are classified as iodine deficient: one country is moderately iodine deficient and 10 countries mildly deficient. Thus there is only one country in which the population would have more than adequate iodine intake and no countries in which the population has excessive intake and would be at-risk of iodine-induced hyperthyroidism. The same is true for some countries classified as having adequate iodine nutrition on a national level, such as Bosnia and Herzegovina, and Serbia and Montenegro, where pockets exist in which people are still affected by iodine deficiency. Spain is a further example where the prevalence of iodine deficiency was previously known to vary markedly between regions (2), with limited areas of severe iodine deficiency complicated by cretinism (92). Nonetheless, it is likely that Spain is still affected by some degree of mild iodine deficiency, considering the absence of a national intervention programme during the past decade. The country has recently announced the implementation of a national iodine deficiency programme. Extrapolating the proportion of school-age children to the general population, it is estimated that 244 million individuals have insufficient iodine intake. They are nationally or subnationally representative in 13 and 10 countries, respectively. It should be noted that subnational data from selected area(s) only represent part of the country. Finally, the present review is essentially based on data published in the scientific literature. This does not include the results of all the investigations that have been carried out, as many have not been published. The concentration of the fortification levels range from 5 ppm (Norway) to 70 ppm (Sweden and Turkey). Information on the present state of legislation is available for 29 countries (Table 3.
Buy 6mg exelon otc. Weird Dehydration Warning Signs and Natural Treatments.