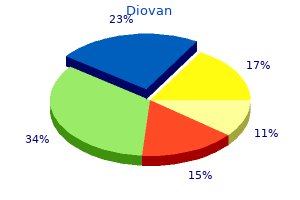
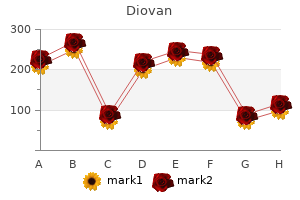
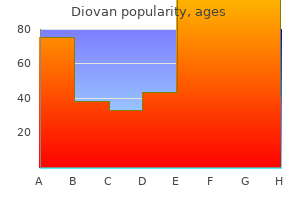
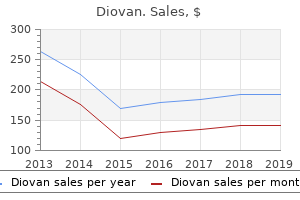
Diovan
"Diovan 40mg fast delivery, arrhythmia nausea".
By: K. Osmund, M.B. B.CH. B.A.O., M.B.B.Ch., Ph.D.
Vice Chair, Louisiana State University
Peripheral vasoconstriction and gross plasma extravasation were reported in a man who accidentally fell into a cistern with hot copper cyanide (Dodds and McKnight 1985) prehypertension effects order 160mg diovan free shipping. Palpitations were recorded in three men who wore respiratory masks while working in an atmosphere containing 20 blood pressure medication low blood pressure order diovan 80mg on-line,000 ppm hydrogen cyanide for 810 minutes (Drinker 1932). No studies were located regarding cardiovascular effects in animals after dermal exposure to cyanide. Acute dermal exposure of guinea pigs to an unknown concentration of hydrogen cyanide resulted in submucous hemorrhages in the stomach as observed at necropsy (Fairley et al. The information regarding renal effects following dermal exposure to cyanide in No studies were located regarding gastrointestinal effects in humans after humans is limited to one case report. Transitory oliguria (scanty urination) was observed in a patient who accidentally fell into a cistern containing 1,000 gallons of hot copper cyanide and remained there for 3 minutes before being rescued (Dodds and McKnight 1985). No studies were located regarding renal effects in animals after dermal exposure to cyanide. No studies were located regarding dermal effects in humans after dermal exposure to When the skin of rabbits was exposed to 5,000 ppm cyanide as cyanogen for 8 hours, no dermal lesions were found (McNerney and Schrenk 1960). Vascular congestion was reported in the skin of guinea pigs after exposure to unknown doses of hydrogen cyanide for 65 minutes (Fairley et al. Irritation, lacrimation, and conjunctival hyperemia were present immediately after the treatment. No studies were located regarding ocular effects in humans after dermal exposure to 3. Similarly, a worker, whose hand was exposed to liquid hydrogen cyanide, fell into a coma, lost deep reflexes, and showed dilated pupils within 5 minutes (Potter 1950). Men working in an atmosphere containing 20,000 ppm hydrogen cyanide for 810 minutes experienced dizziness, weakness, and headaches (Drinker 1932). The workers wore masks that were reported to give excellent respiratory protection. However, exposure to such high concentrations is not safe because the gas is absorbed through the unprotected skin. Similarly, convulsions and coma preceded death in guinea pigs exposed dermally to unknown doses of hydrogen cyanide (Fairley et al. No studies were located regarding the following health effects in humans or animals after dermal exposure to cyanide: 3. No studies were located regarding genotoxic effects in humans after oral or inhalation exposure to cyanide. Adding S-9 mix to the culture decreased the induction of reverse mutations by cyanide to 40% of the nonactivated reaction. The Vitotox test is a screening assay for genotoxicity and cytotoxicity (Merilдinen and Lampinen 2004). Following inhalation, cyanide is rapidly distributed throughout the body, with measurable levels detected in all organs studied to date. Cyanide can be distributed in the body within seconds and death can occur within minutes. Following oral exposure, the highest levels have been detected in the lungs and blood. Animal studies have shown that cyanide does not accumulate in the blood and tissues following chronic oral exposure. Cyanide is transformed to thiocyanate in the body, with a plasma half-life of 20 minutes to 1 hour. Cyanide metabolites are excreted primarily in the urine, with small amounts excreted through the lungs. Humans retained 58% of hydrogen cyanide in the lungs after inhaling the gas through normal breathing (Landahl and Herrmann 1950). Quantitative data on the absorption of hydrogen cyanide by inhalation were reported in dogs (Gettler and Baine 1938). Based on a concentration of 200 mg hydrogen cyanide/L in the blood 2 hours after ingestion, it was estimated that the patient had 1. The gastrointestinal absorption of cyanide following ingestion of certain complex iron-containing cyanide compounds is low because cyanide binds with high affinity to iron.
Please review the information on coordinating benefits with Medicare Advantage plans on page 119 prehypertension vyvanse order genuine diovan online. If you do not sign up for Medicare Part B when you are first eligible hypertension bradycardia generic diovan 160 mg online, you may be charged a Medicare Part B late enrollment penalty of a 10% increase in premium for every 12 months you are not enrolled. Please refer to When you are age 65 or over and do not have Medicare in this section for information about how we provide benefits when you are age 65 or older and do not have Medicare. All physicians and other providers are required by law to file claims directly to Medicare for members with Medicare Part B, when Medicare is primary. When you are enrolled in Original Medicare along with this Plan, you still need to follow the rules in this brochure for us to cover your care. We do not require precertification, prior approval, or concurrent review when Medicare Part A and/or Part B is the primary payor. Precertification, prior approval, and concurrent review are required, however, when Medicare stops paying benefits for any reason. We do not require prior authorization for the purchase of certain prescription drugs when Medicare Part B or Part D is the primary payor for the drugs or you are outside the 50 United States and purchase them from a retail pharmacy outside the 50 United States. However, when Medicare stops paying benefits for any reason, you must follow our precertification, prior approval, prior authorization, and concurrent review procedures. We limit our payment to an amount that supplements the benefits that Medicare would pay under Medicare Part A (Hospital insurance) and Medicare Part B (Medical insurance), regardless of whether Medicare pays. Note: We pay our regular benefits for emergency services to an institutional provider, such as a hospital that does not participate with Medicare and is not reimbursed by Medicare. Claims process when you have the Original Medicare Plan Send us a copy of your Medicare Card when we are secondary to Medicare. Electronic crossover is a process that assures, in most cases, you do not have to file a claim when Medicare is primary. Login to the Member Portal with your username and password to find out if your claims are being electronically filed or you have questions about the process described on the next page. You probably will not need to file a claim form when you have both our Plan and the Original Medicare Plan. In most cases, we will coordinate your claim automatically and provide secondary benefits for covered charges. We waive some costs if the Original Medicare Plan is your primary insurance provider We will waive some out-of-pocket costs as follows: · Medical services and supplies provided by physicians and other health care professionals in Section 5(a). If you purchase Medicare Part B, your provider is in our network and participates in Medicare, then we waive some costs because Medicare will be the primary payor. Benefit Description High Option You pay without Medicare In-Network $300 self only/ $600 family $5,000 self only/ $7,000 family N/A High Option You pay without Medicare Out-of-Network $400 self only/ $800 family $7,000 self only/ $9,000 family N/A High Option You pay with Medicare Part B In-Network $0 $5,000 self only/ $7,000 family N/A High Option You pay with Medicare Part B Out-of-Network $0 $7,000 self only/ $9,000 family N/A Deductible Out of Pocket Maximum Part B Premium Reimbursement Offered Primary Care Physician 10% of the Plan allowance Specialist 10% of the Plan allowance Inpatient Hospital $0 Outpatient Hospital 10% of the Plan allowance Incentives offered · Tell us about your Medicare coverage N/A 30% of the Plan allowance and any difference between our allowance and the billed amount 30% of the Plan allowance and any difference between our allowance and the billed amount $200 per admission and 20% of the Plan allowance and any difference between our allowance and the billed amount 30% of the Plan allowance and any difference between our allowance and the billed amount N/A $0 $0 $0 $0 $0 $0 $200 per admission and 20% of the Plan allowance and any difference between our allowance and the billed amount $0 N/A N/A You must tell us if you or a covered family member has Medicare coverage, and let us obtain information about services denied or paid under Medicare if we ask. You also must tell us about other coverage you or your covered family members may have, as this coverage may affect the primary/secondary status of this Plan and Medicare. Medicare will not pay any portion of the charges and we will not increase our payment. If the physician did not inform you of his/her "Opt Out" status or did not ask you to sign a private contract, we will process your initial claim for that physician using our regular innetwork/out-of-network benefit coinsurance. We will inform you and your physician in a letter that future claims will be processed per the above paragraph. If you continue receiving services from the physician, you will be responsible for paying the difference between the billed amount and the amount we paid as described above. We will need to know whether you are in the Original Medicare Plan or in a Medicare Advantage plan so we can correctly coordinate benefits with Medicare. The following chart illustrates whether Medicare or this Plan should be the primary payor for you according to your employment status and other factors determined by Medicare. However, in certain limited situations, Medicare may be primary for certain types of health care services you receive. Your physician and hospital must follow Medicare rules and cannot bill you for more than they could bill you if you had Medicare. Outpatient hospital care and non-physician based care are not covered by this law; regular Plan benefits apply.
Ans: Yes pulse pressure of 70 diovan 160mg overnight delivery, licensee or heart attack or panic attack diovan 80mg with mastercard, authorized agent in India need to take prior approval from licensing authority in case of change in name and/or address of legal and/or actual manufacturer. Ques: Whether acquisition/merger of one company by another company is considered as change in constitution of the company? Ques: What are the changes that require an applicant to make a fresh import license application? Ans: Fresh import license application shall be made only in case of change of constitution. Ques: Whether the Importer who is having valid import license but there is some change in the name of importer or address of Importer still can he imports till another license is granted. Ques: What is the procedure for expanding/ modifying the currently registered indications? Ans: Expanding or modifying the indications/intended use are considered as a major change under sixth schedule of Medical Device Rules 2017. In case response/approval is not received within 60 days from the notification submission, the products undergone a major change shall be allowed for import. Ques: In case the registered manufacturing site (Actual Manufacturer) remain unchanged (Plant master file to be precise), but Legal manufacturer entity changes to a different entity, whether same Plant Master Files shall be acceptable when submitted towards fresh registration? Ques: Whether authorised agent can submit single application for grant of import licence for same product manufactured at more than one manufacturing sites. Ans: Yes, provided that the applicant shall submit separate fee for each of the sites. Both shelf life or expiry date and date of manufacture shall require on the labels. However, the same shall also be applicable for the products imported under a test license for the purpose of demonstration or training. Ans: Yes; Import License in Form 14 application can be submitted with one lot report, alongwith undertaking of availability of remaining two lots. Stability data Testing protocols for raw materials and finished products In- house specification Labeling Details Evaluation Reports. Ans: the manufacturing company shall submit their application to the State Drugs Control authority under whose Jurisdiction, the manufacturing Premises is located. In critical cases, the said joint inspection team should also co-opt one expert of the related Diagnostic field. Ques: During the validity period of a manufacturing Licence, how much Inspection shall be warranted? Ans: the contents of Plant Master File have been detailed in appendix - I of Fourth schedule. Ans: "loan licence" means a licence issued for manufacturing a medical device by the State Licensing Authority or the Central Licensing Authority, as the case may be, to a person who intends to utilise the manufacturing site of other licencee for manufacturing the same medical device as manufactured by the licencee at that site, Reference Rule-3(Z). Ques: In which form permission to import small quantities of medical devices for personal use can be obtained? Ans: (i) the medical device shall conform to the standards laid down by the Bureau of Indian Standards established under section 3 of the Bureau of Indian Standards Act, 1985 (63 of 1985) or as may be notified by the Ministry of Health and Family Welfare in the Central Government, from time to time. Ans: As per Rule- 44 (e) the date of expiry shall be in terms of the month and the year and it shall mean that the medical device is recommended till the last day of the month and the date of expiry shall be preceded by the words "Expiry date" or "Shelf Life". They are not intended to define a standard of care, and should not be construed as one. Neither should they be interpreted as prescribing an exclusive course of management. Variations in practice will inevitably and appropriately occur when clinicians take into account the needs of individual patients, available resources, and limitations unique to an institution or type of practice. The recommendations for research contained within this document are general and do not imply a specific protocol. Specifically, all members of the Work Group are required to complete, sign, and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest. All affiliations are published in their entirety at the end of this publication in the Biographical Sketch section of the Work Group members. In citing this document, the following format should be used: National Kidney Foundation.
A characteristic sign of a basilar skull fracture is: heart attack jogging purchase line diovan. A brain injury can cause serious brain damage because: 3 blood pressure chart seniors cheap diovan 160mg with visa. Five symptoms of postconcussion syndrome are:, and. After concussion, a patient needs to know to seek medical attention if any of the following six symptoms occur:, and. The most serious brain injury that can develop within the cranial vault is a:. Identify four signs of a rapidly expanding, acute subdural hematoma that would require immediate surgical intervention:, and. The three cardinal signs of brain death are:, and. List five collaborative problems that a nurse should assess for a patient with a brain injury:, and. Name the three criteria used to assess level of consciousness using the Glasgow Coma Scale:, and. Complications after traumatic head injuries can be classified according to:, and. The five vertebrae most commonly involved in spinal cord injuries are the:, and. List four common manifestations of pulmonary embolism:, and. Three potential complications that may develop in spinal cord injury are:, and. Patients with tetraplegia and paraplegia are at increased risk for infection and sepsis from three common sources:, and. Distinguish between three types of intracranial hemorrhage: epidural hematoma, subdural hematoma, and intracerebral hemorrhage. Explain why the most important consideration in any head injury is whether the brain is injured. Emphasize the following areas in your nursing care plan: psychological support, weight-bearing activities, muscle exercises, mobilization, and sexual needs. Nursing Diagnoses Goals Nursing Actions Rationale Expected Outcomes Copyright © 2010 Wolters Kluwer Health/Lippincott Williams & Wilkins. A brain abscess is a collection of infectious material within the substance of the brain that is caused by: a. During assessment, the nurse knows that the most frequently reported disabling symptom found in multiple sclerosis is: a. A positive diagnosis of myasthenia gravis can be reached using the following test: a. A surgical intervention that can cause substantial remission of myasthenia gravis is: a. Tinnitus and vertigo are clinical manifestations of damage to which cranial nerve. Name the five infectious disorders of the nervous system:, and. List six signs of bacterial meningitis that a nurse should assess:, and. The most common cause of acute encephalitis in the United States is:. The two medications of choice for this disorder are: and. The diagnosis of CreutzfeldtJakob disease can now be supported by the presence of: in the cerebrospinal fluid. The primary pathology of multiple sclerosis is damage to the:. List the three forms of multiple sclerosis based on the frequency and progression of symptoms:, and. Myasthenia gravis is considered an autoimmune disease in which antibodies are directed against.
Order diovan 160mg otc. Hypertension Today: JNC-8 Evidence-Based Guidelines.