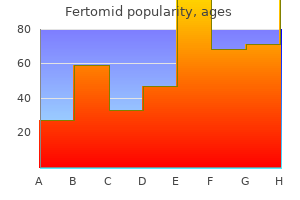
Fertomid
"Purchase fertomid on line, menstrual smell".
By: H. Bandaro, M.B. B.CH., M.B.B.Ch., Ph.D.
Professor, Wayne State University School of Medicine
The traction produced by contracting smooth muscle cells is transmitted to the reticuloelastic sheath about the cells and then to the denser connective tissue women's health center uga cheap fertomid, permitting a uniform contraction throughout the muscle sheet breast cancer walk san diego purchase on line fertomid. The reticular and elastic fibers of the reticuloelastic sheath are products of the smooth muscle cells. Histogenesis Skeletal muscle arises from mesenchyme in various parts of the embryo. Muscles of the limbs and trunk develop from myotomal mesenchyme; those of the head and neck arise in the mesenchyme of the branchial arches. Initially the precursor muscle cells are rounded, noncontractile cells with single centrally placed nuclei. As the cells develop, they elongate and begin to synthesize myofilaments, creatinine kinase, and acetylcholine receptors, thus becoming identifiable as myoblasts. The spindle-shaped myoblasts contain numerous ribosomes, prominent Golgi complexes, and rather short mitochondria, as well as a few myofilaments. Bulk synthesis of contractile proteins occurs in the myotubule, and organization of sarcomeres with recognizable A and I bands and Z lines takes place. Clusters of myofibrils appear, aggregate, increase in length and thicken, and soon show banding. Fusion of Z bodies to which actin filaments are joined results in the appearance of Z lines, aligned with the T-tubule system and units of the sarcoplasmic reticulum. New generations of myoblasts continue to develop and either form new myotubules or fuse to the ends of older myotubules to form fibers that contain 50 to 100 nuclei. It is likely that new myotubules continue to form until their number equals that of the cells of a given mature muscle. By the thirteenth week, the nuclei begin to move from the center of the tube to the periphery, and the cell becomes known as a myofiber. However, development of the cell is not synchronous along its length, and the same structure may show features of a myotubule and a myofiber. The most mature regions are those in the middle of the cell, where fusion of myoblasts first occurred. Thus, for a time the middle of the cell might show peripheral nuclei, while at the ends, central nuclei are present as fusion of myoblasts continues. As the myotubule develops, the number of mitochondria increases and the cristae increase in number and are more tightly packed. There is no evidence that the number of cells increases postnatally, but the cells do increase in length by the addition of new sarcomeres at the ends. Increase in girth is due to an increase in the number of myofibrils and the amount of sarcoplasm. Rather, satellite cells associated with the mature fibers proliferate and fuse to form new skeletal muscle cells. The forming fibers differentiate in a similar way as observed during normal development. Cardiac muscle develops from mesenchyme (the myoepithelial mantle) that envelops the endocardial tube of the embryonic heart. With the synthesis of myofilaments, the cells elongate and become myoblasts, which soon begin rhythmic contractions. Cell division continues into postnatal life but subsequently gives way to hypertrophy as the means of further growth. The sites of desmosomal unions between cells transform into the intercalated discs. Formation of Ttubules characterizes the final stage of development of mammalian cardiac muscle.

The characteristics of the patients in the two groups were well balanced at baseline (Table 1) breast cancer yoga pants generic 50mg fertomid visa. Slightly more patients in the radiotherapy group than in the radiotherapy-plus-temozolomide group were receiving corticosteroids at the time of randomization (75 percent vs menopause center of mn purchase 50 mg fertomid mastercard. A performance status of 0 denotes asymptomatic, 1 symptomatic and fully ambulatory, and 2 symptomatic and in bed less than 50 percent of the day. Unplanned interruptions in radiotherapy were usually brief (median, four days) and interruptions due to the toxicity of therapy occurred in only 3 percent of the radiotherapy group and 4 percent of the radiotherapy-plus-temozolomide group. One patient randomly assigned to radiotherapy alone received radiotherapy plus temozolomide. Among the 287 patients who were assigned to receive concomitant radiotherapy plus temozolomide, 85 percent completed both radiotherapy and temozolomide as planned. Thirty-seven patients (13 percent) prematurely discontinued temozolomide because of toxic effects (in 14 patients), disease progression (in 11), or other reasons (in 12). After radiotherapy, 223 patients in the radiotherapy-plus-temozolomide group (78 percent) started adjuvant temozolomide and received a median of 3 cycles (range, 0 to 7); 47 percent of patients completed 6 cycles. The new england journal of medicine beginning or not completing adjuvant temozolomide therapy was disease progression. Only 8 percent of patients discontinued adjuvant temozolomide because of toxic effects. Beginning with cycle 2, the dose of temozolomide was increased to 200 mg per square meter in 67 percent of patients. Only 9 percent of patients did not receive the higher dose because of hematologic toxicity. The unadjusted hazard ratio for death in the radiotherapy-plus-temozolomide group as compared with the radiotherapy group was 0. These data indicate a 37 percent relative reduction in the risk of death for patients treated with radiotherapy plus temozolomide, as compared with those who received radiotherapy alone. The two-year survival rate was 100 Probability of Overall Survival (%) 90 80 70 60 50 40 30 20 10 0 0 6 12 18 24 30 36 42 Radiotherapy plus temozolomide 26. The hazard ratio for death was adjusted by fitting the Cox proportional-hazard models. The adjusted hazard ratio for death in the radiotherapy-plus-temozolomide group as compared with the radiotherapy group - 0. Radiotherapy plus temozolomide was associated with a significant improvement in median overall survival in nearly all subgroups of patients. The hazard ratio for death among patients treated with radiotherapy plus temozolomide, as compared with those who received radiotherapy alone, was 0. We analyzed adverse events separately during radiotherapy (with or without concomitant temozolomide), the adjuvant-therapy period, and the entire study period (from study entry until disease progression or last follow-up). No grade 3 or 4 hematologic toxic effects were observed in the radiotherapy group. During concomitant temozolomide therapy, grade 3 or 4 neutropenia was documented in 12 patients (4 percent), and grade 3 or 4 thrombocytopenia occurred in 9 patients (3 percent) (Table 4). Overall, 19 patients (7 percent) had any type of grade 3 or 4 hematologic toxic effect. During adjuvant temozolomide therapy, 14 percent of patients 992 n engl j med 352;10 During the radiotherapy period, severe infections occurred in 6 patients in the radiotherapy group (2 percent) and in 9 patients in the radiotherapy-plus-temozolomide group (3 percent); during adjuvant temozolomide therapy, 12 patients (5 percent) had severe infections. The most common nonhematologic adverse event during radiotherapy was moderate-to-severe fatigue in 26 percent of patients in the radiotherapy group and 33 percent in the radiotherapy-plus-temozolomide group (Table 2 in the Supplementary Appendix). Thromboembolic events occurred in 28 patients (5 percent) - 16 in the radiotherapy group and 12 in the radiotherapyplus-temozolomide group. Two patients in the radiotherapy-plus-temozolomide group died of cerebral hemorrhage in the absence of a coagulation disorder or thrombocytopenia.
Cell types and signals that are normally tightly regulated can become de-regulated and co-opted during tumorigenesis breast cancer awareness socks fertomid 50 mg, such that these instructive cues become inappropriately active in response to specific genetic mutations menstrual tent buy fertomid 50 mg overnight delivery. Although Nf1 inactivation in glial lineage cells is a necessary step in oncogenesis, it must occur in a cell type capable of expanding in response to loss of neurofibromin growth regulation. Elegant studies by a number of groups have demonstrated a more limited capacity for accelerated growth following Nf1 inactivation in differentiated astrocytes compared with glial progenitor cells (Zhu et al. Together, these observations establish a regional and developmental context in which bi-allelic Nf1 inactivation will lead to glioma formation. In addition to Nf1 loss in a susceptible cell type, environmental factors are critical determinants of gliomagenesis. These include supportive cell types, such as microglia, reactive astrocytes and endothelial cells. Although the role of microglia in Nf1 glioma formation and growth has gained traction, there are fewer data currently available on the important roles that reactive astrocytes and endothelial cells have. Microglia are known in other pathological conditions to increase endothelial cell migration and proliferation as well as to stimulate reactive gliosis. In this manner, microgliainduced neoangiogenesis might create a supportive niche for cancer stem cells, as has been reported for highgrade gliomas (Ludwig et al. First, it is defined by the presence of cell types and signals unique to a specific region of the brain during a given time of development. In this respect, glial Nf1 loss in the cerebellum occurs in a completely different stromal context than it does in the optic chiasm or brainstem. The gliomagens that facilitate glioma formation and growth as well as the stromagens that promote the establishment of a permissive local environment in one brain region are not equivalent to those present in another brain region. Similarly, the stromagens and gliomagens found in a region at one developmental stage may not be identical to those present later in life. Second, the impact of Nf1 heterozygosity on the evolving tumor microenvironment is unlikely to be the same in all brain locations and at all developmental periods. As different cells in the brain express different molecules in a spatially and temporally regulated fashion, Nf1 heterozygosity may have unique effects on the local brain microenvironment that reflect these regional and developmental conditions. Third, the tumor microenvironment is a dynamic ecosystem, such that the elaboration of specific molecules changes both the cellular and molecular soil in which Nf1-deficient pre-neoplastic/neoplastic cells grow. First, Nf1 inactivation must occur in a preneoplastic cell sensitive to Nf1 loss, such as astroglial progenitors of the optic nerve, to result in increased proliferation, survival and migration. Second, a supportive microenvironment is required to facilitate the expansion of the Nf1-deficient glial cells in a spatially and temporally restricted fashion. This state of flux creates a delicately balanced ecological niche as well as a moving target for therapy (Figure 4). Another level of system complexity results from the influence of the genomic environment. Although not completely elucidated, genetic modifiers likely change the expression of key stromal growth/survival factors or the activity of specific kinases and enzymes. Although these minor alterations by themselves are not sufficient to result in tumor formation, the confluence of these subtle changes in the correct brain region, at the correct time, and in response to specific cancer-initiating genetic changes could significantly influence gliomagenesis and glioma maintenance. Finally, as we move into an era of personalized medicine, it will become increasingly important to consider developing therapies that specifically target the neoplastic and non-neoplastic cells in the tumor and to conceptualize tumors as evolving ecosystems. Using this approach, we may be able to design treatments that ultimately result in durable clinical responses. Similarly, should genomic polymorphisms identify at-risk patient subpopulations, the ability to disrupt tumorigenesis by impeding neoplastic/non-neoplastic cell interactions during cancer evolution may be possible.
Generic fertomid 50mg. Event Trends Expo 2016.