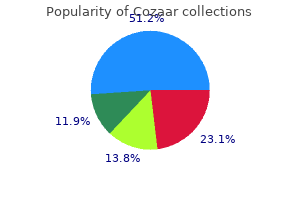
Cozaar
"Generic cozaar 25 mg online, diabetes diet in india".
By: K. Campa, M.B. B.A.O., M.B.B.Ch., Ph.D.
Deputy Director, Charles R. Drew University of Medicine and Science College of Medicine
For example diabetes type 1 impact on health care resources buy cozaar 50 mg line, ~1018 atoms/cm3 of spin-polarized hydrogen atoms embedded in a frozen H2 matrix underneath a superfluid helium film at 0 diabetes medications and heart failure generic cozaar 50mg without prescription. A few years later the same group boosted atomic H density to 2 x 1019 atoms/cm3 with strong suppression of recombination into H2 molecules, which was explained by "the increasing role of energy level mismatch at near distances as the atoms approach each other and the temperature is lowered. Stabilization of hydrogen atoms in aggregates of krypton nanoclusters immersed in superfluid helium. Studies of metallofullerene primary soots by laser and thermal desorption mass spectrometry. Preparation of endohedral complexes of molecular hydrogenfullerene C60, together with hydrogenated C60. Electron structure of endohedrally confined atoms: atomic hydrogen in an attractive shell. Spectral properties of endohedrally confined hydrogen atom and hydrogen-like ions obtained by using B-spline basis set. Theoretical investigation on the encapsulation of atomic hydrogen into heterofullerene nanocages. Hydrogen and deuterium atoms in octasilsesquioxanes: Experimental and computational studies. Cryogenic temperatures may be required if atomic hydrogen in C60 is not stable at room temperature. Atomic hydrogen transmission through five-membered carbon ring by the mechanism of non-adiabatic tunneling. Thus the walls of our physical container that must store the naked protons should consist of a material with the highest possible first ionization potential. That way, electrons will prefer to stay bound to neutral wall atoms and not jump to the naked proton and neutralize it, which would produce a higher total energy for the proton/neutral-atom system. Only 7 elements have higher first ionization potentials than hydrogen (chart, below):585 He (24. The electric potential energy of np protons placed in a box of size Lbox with a mean interproton separation of rmean = (Lbox3/np)1/3 is just the sum of the energies of each unique pair of particles,588 or Ecloud ~ q2 C(np,2) / 40 rmean; setting Ecloud/np E, then Lbox q2 C(np,2) / 40 np2/3E, the minimum box size into which np protons can be packed without exceeding an electric potential energy of E per proton, where proton charge q = 1. For example, a Penning trap stores cold charged particles using a strong homogeneous axial magnetic field to confine particles radially and a 588 the combination number (binomial coefficient) of unique groupings of k items drawn from a total population of n items is C(n,k) = n! Assuming a simple MaxwellBoltzmann distribution, to ensure that fewer than 1 of 207 particles in the box will have an energy of 8 eV or higher requires the group mean electric potential energy should not exceed 1. The prospect of fusion energy has inspired a great deal of experimental effort to confine hot plasma ions (usually deuterium and tritium, not hydrogen) for sufficiently long confinement times to allow nuclear fusion to occur. Comparison of Equilibrium Ion Density Distribution and Trapping Force in Penning, Paul, and Combined Ion Traps. For example, the Sun gravitationally traps a 90% H / 9% He (by atom count) charged ion plasma with a mean density of 1. However, mining these high energy density materials may prove technically challenging. Beam densities in excess of ~1018 protons/cm3 are probably required for a practical molecular recombination energy storage system. To this end, it has been asserted that metallic hydrogen would make an ideal high-energy rocket fuel. Instead of discrete H2 molecules having two electrons bound between two protons, a bulk phase would form consisting of a liquid or solid lattice of protons, with electrons delocalized throughout as occurs in conductive metals. Quartzlike carbon dioxide: an optically nonlinear extended solid at high pressures and temperatures. Role of electron-electron interaction in the formation of a metastable state of metallic hydrogen. The most straightforward technique for making liquid metallic hydrogen is simple compression of molecular hydrogen at constant temperature (moving from left to right in the phase diagram above). Over the decades there have been many claimed observations of metallic hydrogen,639 though none have yet been widely accepted by the high-pressure physics community. Evidence for a first-order liquid-liquid transition in high-pressure hydrogen from ab initio simulations.
It may be necessary to process raw materials from a source to convert it to the specific material used as a feedstock for a chemical process diabetes in labrador dogs discount cozaar online. Often most of the environmental harm in providing feedstocks comes during the isolation process diabetes insipidus with lithium 50 mg cozaar sale, in large part because of the relatively large amount of waste material that must be discarded in obtaining the needed feedstock. Once a suitable chemical feedstock is obtained, it is subjected to chemical processes that give the final product. As addressed below, this may consist of reactions with various kinds of reagents in media such as organic solvents, often using catalysts. Reagents the term reagents is used here to describe the substances that act upon basic chemical feedstocks to convert them to new chemicals in synthetic processes. The kinds of reagents used have a very strong effect upon the acceptability of a chemical process with respect to green chemical aspects. Much of the work that has been done in developing and using green reagents has involved organic chemical processes, many of which are beyond the scope of this book. However, some of the general aspects of chemical reagents from a green chemical perspective are discussed here. The most obvious characteristic required of a good chemical reagent is that it do what it is supposed to do, completely, and at an acceptable rate. A reagent with a high product selectivity produces a high percent age of the desired product with a low percentage of undesired byproducts. Another desirable characteristic of a good reagent is high product yield meaning that most of the feedstocks are converted to product. The use of reagents that provide high selectivity and yield means that less unreacted feedstock and byproduct material have to be handled or disposed. One of the most common measures taken in implementing green chemical processes is selection of alternative reagents. The criteria used in selecting a reagent include whether or not it is available, how efficient it is, and its effects. Important considerations with the chemical transformation are whether it is stoichiometric or catalytic, the degree to which it is atom economical, and the quantities and characteristics of any wastes produced. One of the main kinds of reactions for which reagents are used is oxidation, which usually consists of the addition of oxygen to a chemical compound or a functional group on a compound. Some of these reagents, such as potassium dichromate, K2Cr2O7 are dangerous (dichromate salts are considered to be carcinogenic when inhaled for prolonged periods of time) and leave troublesome residues that require disposal. Because of problems with oxidants that are commonly used, a major objective in the practice of green chemistry is to use more benign oxidants. Alternatives to the more traditional oxidant reagents include molecular oxygen (O2), ozone (O3), and hydrogen peroxide (H2O2), usually used with a suitable catalyst that enables the oxidation reaction to occur. Under the right conditions, hydrogen peroxide can be used as an alternative to elemental chlorine, Cl2, a strong oxidant used in bleaching colored materials, such as paper pulp and cloth. Since chlorine is toxic (it was used as a poison gas in World War I) and has a tendency to react with organic compounds to produce undesirable chlorinated organic compounds, hydrogen peroxide is a much preferable bleaching agent. In contrast to the usually harsh conditions under which chemical oxidations are carried out, organisms carry out biochemical oxidations under mild conditions. In so doing, they use monooxygenase and peroxidase enzymes that catalyze the oxidizing action of molecular oxygen or hydrogen peroxide. An area of significant interest in green chemistry is to perform such oxidations in biological systems or to attempt the use of catalysts that mimic the action of enzymes in catalyzing oxidations with molecular oxygen or hydrogen peroxide. Reduction, which consists of loss of O, gain of H, or gain of electrons by a chemical species is also a common operation in chemical synthesis. As is the case with oxidants, the reagents used to accomplish reduction can pose hazards and produce undesirable byproducts. Such reductants include lithium aluminum hydride (LiAlH4) and tributyl tin hydride. As an alternative to the potentially troublesome oxidation and reduction procedures using reagents, electrochemistry provides a reagentless means of doing oxidation and reduction. This is possible because an electrical current consists of moving electrons and oxidation consists of electron removal from a chemical species and reduction is addition of an electron. The passage of an electrical current between metal or carbon graphite electrodes through a solution resulting in oxidation and reduction reactions is called electrolysis. Toward a Greener Anthrosphere through Industrial Ecology 299 and at the anode where electrons are removed, O2 is released as the water is oxidized: 2H2O O2 + 4H+ + 4e(11. At the cathode, a dissolved chemical species could be reduced directly or the hydrogen generated could add to a species, reducing it.
Two of the largest historic tantalum producers are the Greenbushes and Wodgina deposits in Western Australia diabetes insipidus jurnal order cozaar uk. The Greenbushes pegmatite diabetes definition hemoglobin a1c generic 25 mg cozaar amex, in the Yilgarn Craton, contains Measured, Indicated and Inferred Resources of 135. It is a Spodumene Subtype pegmatite that intruded into a shear separated from niobium (which is an order of magnitude higher in concentration) or from the other heavy minerals. It contains arfvedsonite and aegirine as well as a wide range of accessory minerals including pyrochlore, zircon, smarskite, aeschynite-(Y), columbotantalite and cassiterite. The second type of deposit hosted by peralkaline rocks are those hosted by syenites. There are a variety of different types of nepheline syenites that are mineralised and commonly these intrusions are well layered. These deposits are primarily of interest for their rare earth element mineralisation and will not be discussed in detail. The most common niobium mineral in these deposits is pyrochlore, although at Nechalacho the dominant niobium mineral is fergusonite (see Salvi and WilliamsJones, 2005, for a review). The Motzfeldt Complex in Greenland is an exception, and here, niobium and tantalum are the primary commodities. Peraluminous pegmatites Peraluminous pegmatites have historically been the most important source of tantalum. Many of these pegmatites have been mined intermittently and may or may not be in current production. They are described here because they are well documented and may resume tantalum production in the future. They are commonly associated Tantalum and niobium 369 1100 1000 900 800 700 600 500 0 100 200 300 400 500 400 Distance in feet Zone 20 Wall zone Ta Li Ta Zone 30 Aplitic Albite zone Zone 40 Lower intermediate zone Zone 50 Upper intermediate zone Zone 60 Central intermediate zone Cs Ta Elevation in feet above sea level Zone 70 Quartz zone Zone 80 Pollucite zone Zone 90 Lepidolite zone Amphibolite Xenolith Figure 15. Tantalum mineralisation is contained in the banded aplite (red and blue-grey at the bottom). A massive quartz zone (dark grey) is at the top and coarse, crystalline white beryl separates the aplite and quartz. Cassiterite is also an important ore mineral and early formed tantalum minerals occur as inclusions in cassiterite and tourmaline (Partington et al. Tantalum-bearing pegmatites in Brazil are numerous and widely distributed, but are typically relatively small (less than one million pounds tantalum in size) and are generally mined on a semi-industrial or even artisanal scale. However, the Mibra (Volta) mine, near the city of Nazareno in the state of Minas Gerais, hosts a series of pegmatites, with a total reported (indicated) resource of 25. There are a number of flat, subhorizontal sills, the largest of which measured 1000 by 150 by 20 m before exploitation. The Yichun deposit, China, reported to contain approximately 175 million tonnes (personal communication, 1996), is a vertically zoned granite occupying the top of the south-eastern section of the Yashan granite batholith. The upper portion of the granite, which carries the majority of the tantalum values, is an albitic granite high in lepidolite and topaz, relatively low in quartz, and depleted in dark minerals.
Syndromes
- Antidepressants such as amitriptyline or venlafaxine
- Fecal or anal (the feces is discharged through an opening other than the anus)
- Cosmetic products
- A-200
- Agitation
- Yellowing of the skin (jaundice) -- may come and go
The hydrocarbon terpenes that occur in rubber trees can be tapped from the trees as a latex suspension in tree sap diabetes 66 discount cozaar generic. Steam treatment and distillation can be employed to extract terpenes from sources such as pine or citrus tree biomass diabetes diet kerala menu generic 25 mg cozaar amex. Grain seeds are rich sources of protein, almost always used for food, but potentially useful as chemical feedstocks for specialty applications. An exciting possibility just now coming to fruition in a practical sense is to transplant genes into plants so that they will make specialty proteins, such as medicinal agents. One of these consists of plants, which make huge quantities of cellulose and lesser quantities of other materials by photosynthesis. Fermentation Fermentation refers to the action of microorganisms on nutrients under controlled conditions to produce desired products. Fermentation for some products is anaerobic (absence of air) and for others aerobic fermentation is used. Fermentation processes have been used for thousands of years to produce alcoholic beverages, sauerkraut, vinegar, pickles, cheese, yogurt, and other foods. Ethanol, the alcohol in alcoholic beverages, is the most widely produced chemical made by fermentation. More recently, fermentation has been applied to the production of a wide variety of organic acids, antibiotics, enzymes, and vitamins. Starting in the 1940s, one of the major products of industrial fermentation has been penicillin, of which there are several forms. Following penicillin, fermentation processes were developed for the production of several other significant antibiotics. Selection of the appropriate microorganism is the most important consideration of a successful fermentation production process. The microorganisms have to have the proper nutrients, the choice of which can affect the kind and yield of the product. The temperature of fermentation is important, with fermentation rates increasing up to an optimum temperature, after which they decrease sharply with 310Green Chemistry, 2nd ed increased temperatures as the enzymes used by the microorganisms are destroyed (denatured). This kind of temperature relationship has increased interest in the use of thermophilic microorganisms that exist at boiling water temperatures in hot springs. If such organisms can be engineered to produce desired products, the rate of product generation may increase markedly. Both the levels of oxygen (which must be excluded from anaerobic processes) and pH must be controlled precisely. Modern fermentation processes use a variety of sensors to continuously monitor conditions in the fermentation tank and computerized control to accurately control all the parameters. Penicillium chrysogeum inoculum Mixing, transfer Fermentation tank Filter Extractor Nutrients Evaporatedliquid Extractor Product Vacuumdrying Vacuumdistillation Slurryofcrystallized solid Filter Figure 12. Simplified schematic diagram of the process for making penicillin by fermentation. Fermentation is undergoing tremendous development with the use of transgenic microorganisms to which genes have been transferred to make specific kinds of substances. The most common and valuable substances made by transgenic microorganisms consist of a variety of proteins. These include proteins and smaller molecule polypeptides that Purificationcolumn Chap. The best example of such a substance is human insulin, which is now produced in large quantities by transgenic microorganisms. Until recently, fermentation has not been widely employed to make commodity chemicals used on a large scale. An exception is the large-scale production of ethanol from the fermentation of glucose sugar by yeasts. It is not clear that this is a truly green technology and some authorities believe that the energy consumed and the environmental damage from more intensive cultivation of corn outweigh the benefits of using this grain to produce ethanol fuel. Advances in transgenic microbiology have now raised the possibility of using fermentation for the production of a variety of chemicals and chemical feedstocks, several examples of which are discussed in this chapter. ProductionofMaterialsbyPlants the uses of microorganisms operating in fermentation processes to generate commodity chemicals were discussed above.
Generic cozaar 50 mg on-line. Dr. Tunis Hunt discusses Diabetes Reversal.