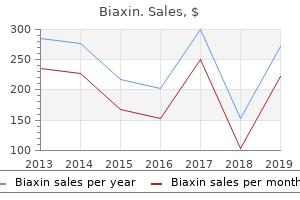
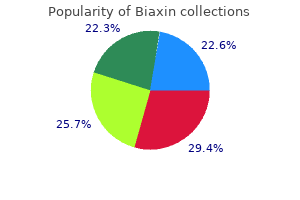
Biaxin
"Purchase biaxin once a day, gastritis symptoms how long does it last".
By: Q. Jensgar, M.A., Ph.D.
Professor, Charles R. Drew University of Medicine and Science
In their current state gastritis kod pasa discount biaxin 250mg visa, we feel the guidance violates all aspects of the triple aim gastritis diet ��� discount 250mg biaxin otc. Therefore, we ask that you seriously consider the additions/changes to the guidelines below. Before getting to those specific details, there are other vital points we ask that you also consider. Once any changes to the Prioritized List are complete, rule changes would need to be made. G 4 Patients who refuse to adhere the guidelines needs to sign an informed refusal consent form. All women giving birth out of hospital should have a full informed consent procedure. System characteristics associated with safe out of hospital birth include a system of consultation and referral/transfer that can assure seamless care. Written agreements that cover consultation/referral/transfer and a welldefined and practiced system of transfer are important as noted in the coverage guidance document. Our past experience has proven that transfer plans are poor at best, and significantly contribute to the maternal/fetal morbidity and mortality. A "well-defined system of transfer" is in the document but no longer in the box language as a characteristic of a successful home birth. Other types of maternity care providers, including midwives as well as family physicians, are qualified to assess dating, maternal history, and infectious disease screening. Complications of pregnancy necessitating consultation or transfer are listed in the box language. Urine toxicology screening may be appropriate in some patients at higher risk but is not universally recommended. Some of these labs may not be obtained due to a variety of factors including patient preference. Inadequate prenatal care may be a proxy for measurement, and women may refuse one or more of these tests. G 13 Below are our other recommendation that would negate a home birth or require transfer to a hospital: Lacerations-see comments F9, F24. However, qualified providers in Oregon may administer group B strep prophylaxis outside the hospital setting and so this is not by itself a high-risk coverage exclusion criterion for out-of-hospital birth. Maternal seizure disorder: Netherlands B if medicated; should indicate consultation prior to planned home birth. Fetal macrosomia is added as an criterion for consultation prior to planned home birth Drug or alcohol use with high risk for adverse effects to fetal or maternal health and mental health disorder requiring inpatient care are listed in box language as high-risk coverage exclusion criteria. Maternal mental illness under outpatient psychiatric care is a criterion for consultation. We admit that we have dealt with past disastrous maternal/fetal outcomes, and as such we feel very strongly about this issue. Again, in their current state, we feel the guidelines violate all three aspects of the triple aim. There should be at least one person present at the delivery whose primary responsibility is the care of the newborn infant and who has the appropriate training, skills and equipment to perform a full resuscitation of the infant. All medical equipment, and the telephone, should be tested before the delivery, and the weather should be monitored. A previous arrangement needs to be made with a medical facility to ensure a safe and timely transport in the event of an emergency. If warranted, infants may also require monitoring for group B streptococcal disease and glucose screening. H 2 In practice, the manner by which infants are assessed for their candidacy for planned home birth is sometimes of concern.
Comparison 1 Total intravenous corticosteroid dose more than or equal to 3000 mg gastritis definition wikipedia generic biaxin 250mg amex, Outcome 4 Participants with normal contrast sensitivity at 6 months gastroenteritis flu cheap biaxin 500 mg otc. Comparison 1 Total intravenous corticosteroid dose more than or equal to 3000 mg, Outcome 5 Participants with normal contrast sensitivity at 1 year. Comparison 1 Total intravenous corticosteroid dose more than or equal to 3000 mg, Outcome 6 Participants with normal contrast sensitivity at 1 month. Comparison 1 Total intravenous corticosteroid dose more than or equal to 3000 mg, Outcome 7 Participants with normal visual field at 6 months. Comparison 1 Total intravenous corticosteroid dose more than or equal to 3000 mg, Outcome 8 Participants with normal visual field at 1 year. Comparison 1 Total intravenous corticosteroid dose more than or equal to 3000 mg, Outcome 9 Participants with normal visual field at 1 month. Comparison 2 Oral corticosteroids versus placebo, Outcome 1 Participants with normal visual acuity. Comparison 2 Oral corticosteroids versus placebo, Outcome 2 Participants with normal contrast sensitivity. Comparison 2 Oral corticosteroids versus placebo, Outcome 3 Participants with normal visual field. Publication status and date: New search for studies and content updated (no change to conclusions), published in Issue 4, 2012. Usually presenting with an abrupt loss of vision, recovery of vision is almost never complete. Closely linked in pathogenesis to multiple sclerosis, it may be the initial manifestation for this condition. Objectives to assess the effects of corticosteroids on visual recovery of patients with acute optic neuritis. There were no date or language restrictions in the electronic searches for trials. We also searched reference lists of identified trial reports to find additional trials. Selection criteria We included randomized trials that evaluated corticosteroids, in any form, dose or route of administration, in people with acute optic neuritis. Data collection and analysis Two authors independently extracted the data on methodological quality and outcomes for analysis. Main results We included six randomized trials which included a total of 750 participants. Two trials evaluated low dose oral corticosteroids while one trial evaluated low dose intravenous corticosteroids across two treatment arms and two trials evaluated a higher dose of intravenous corticosteroids. One three-arm trial evaluated low-dose oral corticosteroids and high-dose intravenous corticosteroids against placebo. Trials evaluating oral corticosteroids compared varying doses of corticosteroids with placebo. We did not conduct a meta-analysis for this outcome at one year follow up since there was substantial statistical heterogeneity. The objective of this review was to assess the effectiveness of corticosteroids for acute optic neuritis. These trials compared corticosteroid therapy to placebo or other treatment with variations in the route of administration and dose administered. At six months and one year, participants randomized to corticosteroids were more likely to have normal vision, contrast sensitivity (ability to distinguish fine changes in the shading of letters on an eye chart), and visual field (area visible when looking straight ahead) compared to participants receiving placebo, but these differences were not clinically meaningful. Adverse effects, although not consistently reported, included acne, high blood sugar, gastrointestinal problems, headache, fever, and sleep and mood disturbances. The findings suggest that there is no evidence of benefit with either oral or intravenous corticosteroids compared to placebo for the outcomes visual acuity, visual field, and contrast sensitivity. It is second only to glaucoma as the most common acquired optic nerve disorder in persons under 50 years. The majority of patients with optic neuritis are between the ages of 20 and 50 years, with a mean age around 30 to 35 years. Koch-Henriksen and Hyllested reported an annual incidence of 4 to 5/100,000 for new onset optic neuritis cases in Denmark in 1948 to 1964 (Koch-Henriksen 1988).
It is possible that clinical heterogeneity in subject populations and small sample sizes may have masked statistically significant effects of milk thistle in different clinical groups gastritis pdf purchase discount biaxin. As we conducted multiple exploratory analyses diet gastritis kronik purchase cheapest biaxin, it is also possible that some of the statistically significant findings occurred by chance. First, searching for studies that report adverse effects is difficult and, given current indexing systems for electronic databases, probably incomplete. Many studies may mention adverse effects in passing, but they do not use adverse effects as a key index word or in their abstracts. If these studies do not otherwise meet selection criteria in a review, they will be missed. Second, information is frequently missing on whether adverse effects were elicited by voluntary selfreport or standardized probes. Third, in some case reports and case series, adverse effects cannot be directly attributed to milk thistle because chance, coincidence, or confounding factors could have been responsible. Fourth, case reports and case series may miss delayed adverse reactions because such associations are more difficult to make than those that occur immediately after administration of an agent. Fifth, although case reports and case series can provide qualitative information about the nature of an adverse effect, they cannot generate estimates of incidence. Seventeen reports of adverse effects possibly due or attributed to milk thistle oral supplements were identified (Evidence Table 5). Data were abstracted from seven randomized clinical trials,66,68,75,77,83,85,87 six cohort studies,88-93 and five case reports. An overall summary of reported adverse effects, by level of evidence, is shown in Summary Table 12. Common Symptomatic Effects Gastrointestinal side effects are the most commonly reported adverse effects, in both randomized clinical trials and prospective cohort studies. In one case report, a variety of symptoms plus "collapse" was associated with milk thistle, improved after discontinuation of milk thistle, reappeared on rechallenge, but was also associated with potential alternative etiologies. Outcome measures and followup times for trials of chronic liver disease, chronic liver disease of mixed etiology, and viral liver disease (continued) Study Outcomes Measures Time Point(s)a,b Chronic viral hepatitis Buzelli79 Kiesewetter80: Reported as improvement, no change, worse Note: When there was more than one study, effect sizes were pooled. Effect sizes and meta-analysis for chronic alcoholic liver disease (6 studies) p Valuec Time of No. Effect sizes and meta-analysis for chronic alcoholic liver disease (6 studies) (continued) p-Valuec Time of No. Effect sizes and meta-analysis for chronic liver disease of mixed etiologies (6 studies) p Valuec Time of No. Effect sizes and meta-analysis for viral liver disease, acute and chronic (3 studies) p Valuec Time of No. All studies refer to combination of chronic alcohol, chronic and acute viral, and chronic mixed etiology unless otherwise noted (the inclusion of one drug study, factorial design); values reported for meta-analytic results; N/A for single study estimates. Conclusions the effectiveness of milk thistle in human liver disease has not been established. This may be because of the scientific quality of study methods or published reports or both. Possible benefit has been shown most frequently, but not consistently, for improvement in aminotransferases, but liver function tests are overwhelmingly the most common outcome measure studied. Mechanisms of action, disease populations likely to benefit, the optimal formulations of milk thistle, and duration of therapy are undefined. Further study of mechanisms of action and well-designed clinical trials, with detailed reporting of adverse effects and components of potential causality, are needed. Future Research Adverse Effects the type, frequency, and severity of adverse effects related to milk thistle preparations should be quantified. When adverse effects are reported, concomitant use of other medications and product content analysis should also be reported so that other drugs, excipients, or contaminants may be scrutinized as potential causal factors. The most serious potential adverse effects of milk thistle reported thus far are anaphylactoid reactions and anaphylaxis, and available data are extremely limited regarding causality. Beneficial Effects Regarding Liver Diseases Studies in humans with physiologic outcomes are limited by unclear study populations, randomization procedures, small sample sizes, variable and sometimes short duration of treatment, unclear blinding for outcome assessment, and unclear or inadequate information about potential confounders. Characteristics of future studies should include longer and larger randomized trials; clinical, as well as physiologic, outcome measures; histologic outcomes; adequate blinding; detailed data about compliance and dropouts; systematic standardized surveillance for adverse effects; and sophisticated considerations regarding study populations and potential confounders. There also should be detailed attention to preparation, standardization, and bioavailability of different formulations of milk thistle. After correction for baseline risk differences, our meta-analyses found generally adequate statistical homogeneity, despite poorly defined and heterogeneous study populations.
Purchase biaxin 250 mg with visa. Gastritis and GERD Friendly Diet.